Why are alkanes and alkenes hydrocarbons?
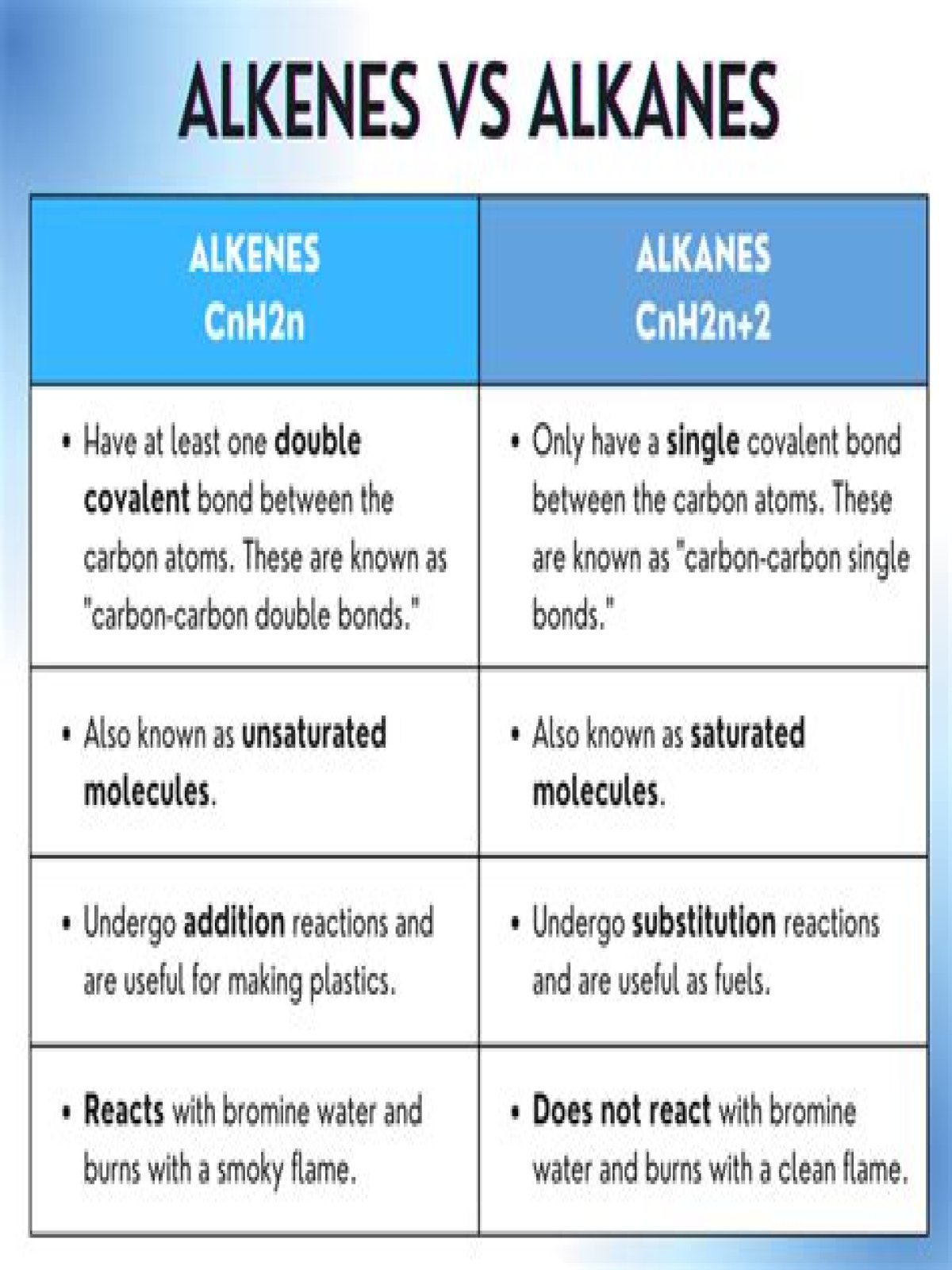
Hydrocarbons are organic compounds composed of only carbon and hydrogen. The alkanes are saturated hydrocarbons—that is, hydrocarbons that contain only single bonds. Alkenes contain one or more carbon-carbon double bonds.
- Why are alkanes described as hydrocarbons?
- Why alkenes are hydrocarbons?
- Are alkenes and alkanes both hydrocarbons?
- What is the main difference between alkanes and alkenes?
- What is more reactive alkanes or alkenes give reasons?
- Why are alkanes less reactive than alkenes?
- Why is Octane an alkane?
- Why are alkenes unsaturated hydrocarbons?
- Why are alkanes saturated and alkenes unsaturated?
- Why are alkanes used as fuels?
- Why are alkanes saturated hydrocarbons?
- Why don't we use alkenes as fuels?
- How do you know if a hydrocarbon is an alkane or alkene?
- Is octane a Mexican?
- Why aromatics have higher octane number?
- What is the old name of alkanes?
- Why does alkane Show least reactivity?
- Why alkene and alkyne is reactive compound?
- Why benzene is less reactive than alkenes?
- Why do you suppose hydrocarbons with double or triple bonds are called unsaturated?
- Why are alkanes more reactive?
- How you can differentiate between an alkane alkene and aromatic compound?
- Is alkene a saturated hydrocarbons?
Why are alkanes described as hydrocarbons?
The alkanes are saturated hydrocarbons: hydrocarbons , because they are compounds containing hydrogen and carbon only. saturated , because their carbon atoms are joined by C-C single bonds.
Why alkenes are hydrocarbons?
Alkenes are a homologous series of hydrocarbons that contain a carbon-carbon double bond. The number of hydrogen atoms in an alkene is double the number of carbon atoms, so they have the general formula C n H 2 n . For example, the molecular formula of ethene is C 2 H 4 , while for propene it is C 3 H 6 .
Are alkenes and alkanes both hydrocarbons?
While alkanes and alkenes are both hydrocarbons, the primary difference is that alkanes are saturated molecules, containing only single covalent bonds (σ-bonds) between the carbon atoms whereas alkenes are unsaturated molecules containing a double covalent bond (combination of a π-bond and a σ-bond).
What is the main difference between alkanes and alkenes?
The main differences between alkanes and alkene are their functional groups and degree of unsaturation. Alkanes are known as saturated hydrocarbons. Alkenes are known unsaturated hydrocarbons as it contains a C=C bond in its structure. The C=C is its functional group.
What is more reactive alkanes or alkenes give reasons?
Alkenes are relatively stable compounds, but are more reactive than alkanes because of the reactivity of the carbon–carbon π-bond. Most reactions of alkenes involve additions to this π bond, forming new single bonds. The carbon-carbon double bond in alkenes such as ethene react with concentrated sulfuric acid.
Why are alkanes less reactive than alkenes?
Alkenes and alkynes are unsaturated hydrocarbons with atleast one double bond which is a Π bond, whereas alkanes contain only σ bonds. As σ bonds are stronger than Π bonds, alkanes are less reactive than alkenes and alkynes.
Why is Octane an alkane?
Octane belongs to a family of molecules called alkanes. These are hydrocarbon molecules with just single, two-electron, bonds connecting the atoms. There is a whole series of them with increasing numbers of carbon atoms, starting from methane (1 carbon), ethane (2 carbons), propane (3), butane (4), etc.
Why are alkenes unsaturated hydrocarbons?
The alkenes are unsaturated hydrocarbons: hydrocarbons , because they are compounds containing hydrogen and carbon only. unsaturated, because they contain a C=C double bond, which means that they have two fewer hydrogen atoms than the corresponding alkane.
Why are alkanes saturated and alkenes unsaturated?
Alkanes are saturated because they contain only single covalent bonds. Alkenes are unsaturated because they do have double covalent bonds.
Why are alkanes used as fuels?
Alkanes will react with Oxygen if they are given sufficient Activation Energy. This will result in a highly Exothermic reaction, producing Carbon Dioxide and Water, which makes Alkanes very useful as fuels.
Why are alkanes saturated hydrocarbons?
The alkanes are saturated hydrocarbons: hydrocarbons , because they are compounds containing hydrogen and carbon only. saturated , because their carbon atoms are joined by C-C single bonds only.
Why don't we use alkenes as fuels?
Alkenes readily burn, just like alkanes, to give carbon dioxide and water if combustion is complete e.g. However, they are NOT used as fuels for two reasons. They are far too valuable for use to make plastics, anti–freeze and numerous other useful compounds.
How do you know if a hydrocarbon is an alkane or alkene?
Alkanes have only single bonds between carbon atoms and are called saturated hydrocarbons. Alkenes have at least one carbon-carbon double bond.
Is octane a Mexican?
Octane, whose real name is Octavio Silva, hails from the world of Psamathe. Although Respawn hasn't confirmed the character's ethnicity, the surname “Silva” is generally of Brazilian or Portuguese descent.
Why aromatics have higher octane number?
the quest for octane
Octane is related to the molecular composition of the fuel. Straight chain paraffins and naphthenes (saturated cyclic hydrocarbons) have poor octane ratings whereas aromatics, branched paraffins and olefins, have high octane values.
What is the old name of alkanes?
The trivial (non-systematic) name for alkanes is 'paraffins'. Together, alkanes are known as the 'paraffin series'. Trivial names for compounds are usually historical artifacts.
Why does alkane Show least reactivity?
Alkanes are saturated and have stronger intermolecular forces of attraction. Thus, a lot of energy is needed to break their bonds. Therefore, they are less reactive.
Why alkene and alkyne is reactive compound?
Alkenes and alkynes are generally more reactive than alkanes due to the electron density available in their pi bonds. In particular, these molecules can participate in a variety of addition reactions and can be used in polymer formation.
Why benzene is less reactive than alkenes?
Substitution of a hydrogen, on the other hand, keeps the aromatic ring intact. That is why benzene less reactive towards electrophiles than an alkene, even though it has more pie lectrons than an alkene(six versus two)
Why do you suppose hydrocarbons with double or triple bonds are called unsaturated?
Carbon atoms with double or triple bonds are unable to bond with as many hydrogen atoms as they could if they were joined only by single bonds. This makes them unsaturated with hydrogen atoms.
Why are alkanes more reactive?
This is because the carbon carbon double bond is a centre for high electron density and so can be attacked by an electrophile (an ion or molecule that attacks regions of high electron density) which will break the bond. The carbon carbon double bond is made up of a pi bond and a sigma bond.
How you can differentiate between an alkane alkene and aromatic compound?
The alkanes are saturated hydrocarbons—that is, hydrocarbons that contain only single bonds. Alkenes contain one or more carbon-carbon double bonds. Alkynes contain one or more carbon-carbon triple bonds. Aromatic hydrocarbons contain ring structures with delocalized π electron systems.
Is alkene a saturated hydrocarbons?
The alkenes are unsaturated. This means that they have a carbon to carbon double bond. The alkanes are saturated because they only contain single bonds.